


Galileo has two key technical advantages. First is its enabling Cell Energy Discovery™ Platform that integrates high throughput screening technologies with both proprietary cell models of human metabolism and dynamic kinetic assays. This platform expedites identifying metabolic nutritional and pharmaceutical targets and enables lead identification and optimization. Second is the Company's novel medicinal chemistry effort directed towards fundamentally new classes of compounds.
Galileo's Energetically Competent™ cell lines accurately model human metabolism, dramatically improving our ability to qualify bioactive candidates that can prevent or treat metabolic and oxidative stress. At the core of Galileo's research and development program is its proprietary Cell Energy Discovery Platform™. This platform successfully links Galileo's EC2™ cell lines with assays and robotic technologies to create an integrated system that allows millions of diverse potential nutritional and pharmaceutical compositions to be evaluated for beneficial properties. Galileo has an extensive portfolio of proprietary natural products and synthetic compositions in various stages of development for its nutritional and pharmaceutical development programs. Galileo has several nutritional product candidates in or about to enter clinical trials targeting the inflammatory components of cardiovascular disease, perimenopausal dyscrasias, and renal failure. In its heart attack and stroke pharmaceutical programs, Galileo will use human bioactivity data and biomarker response profiles from its clinical nutrition programs in conjunction with preclinical pharma data and will complete analogues and PKADME/tox to underpin an IND filing.
|
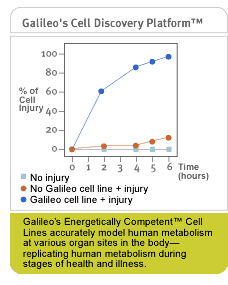 Galileo's
proprietary cell lines accurately model human metabolism at various
organ sites in the body. Using these realistic models, Galileo can
identify points of metabolic vulnerability and the prophylactic
or therapeutic impact of product candidates. These proprietary models,
called Energetically Competent™ cell lines (EC2™),
are unique inasmuch as they model vital organs metabolism.
Galileo's
proprietary cell lines accurately model human metabolism at various
organ sites in the body. Using these realistic models, Galileo can
identify points of metabolic vulnerability and the prophylactic
or therapeutic impact of product candidates. These proprietary models,
called Energetically Competent™ cell lines (EC2™),
are unique inasmuch as they model vital organs metabolism.